The potential use of data from Flatiron to support regulatory and reimbursement applications in oncology has stirred up quite an interest in the field. The current developments we have been seeing prove the potential of usage of such data in the reduction of regulatory and reimbursement approval time.
We believe that it is possible to do similar and sometimes, even better real-world evidence studies in Sweden, despite the small size of the population.
Obviously, the US data has higher validity for a regulatory and reimbursement process in the US. However, the Swedish data, on the other hand, has higher validity in Sweden and it could even serve as a more relevant comparator from a European perspective than a US data-set considering differences in treatment practices and healthcare system difference between US and Europe.
We are currently doing an internal project to illustrate why we believe that this may be a case which needs more attention and discussion. We will share insights from our analysis throughout this project phase. We hope to trigger a healthy discussion and an even better understanding about the choice of most relevant real-world data sources for oncology.
In this first blog-post in the series, we provide an overview of the oncology specific registries in Sweden.
- Linkage and completeness of medical history
With the use of the social civic identity number, it is possible to link the specific oncology registries to all other medical data sets, demographic data of a particular patient as well as rich socioeconomic information which gives a possibility that has been well maintained in Sweden. to follow a patient from birth to death with all events being captured with the possibility of linking all data points with no loss of data. Though this will be further discussed in detail in our upcoming blogs, it is essential to understand this vast coverage and data availability when it comes to the Swedish data sets right from the start.
- Structured data and defined variables
The Swedish oncology registries have been initiated by physicians who want to collect data for research and have defined the relevant variables jointly as a clinical community for each type of cancer. As a result, you can see the richness of variables for each of the data-sets, with an average of more than 400 variables in the registries. There is a variation in the variables collected depending on the specific demand for each type of cancer across the registries. The completeness of the data is usually on par with the quality of a clinical trial. It can be surprising to many and can invoke comments on the possibility of a similarly diligent data-collection method in their country. This can be attributed to the long-standing culture of documenting that has been established in Sweden for centuries which makes this possible. Before disregarding the possibility of developing and executing such a rich and complete data-set, we encourage you to take a deeper look and to evaluate if it might actually be possible.
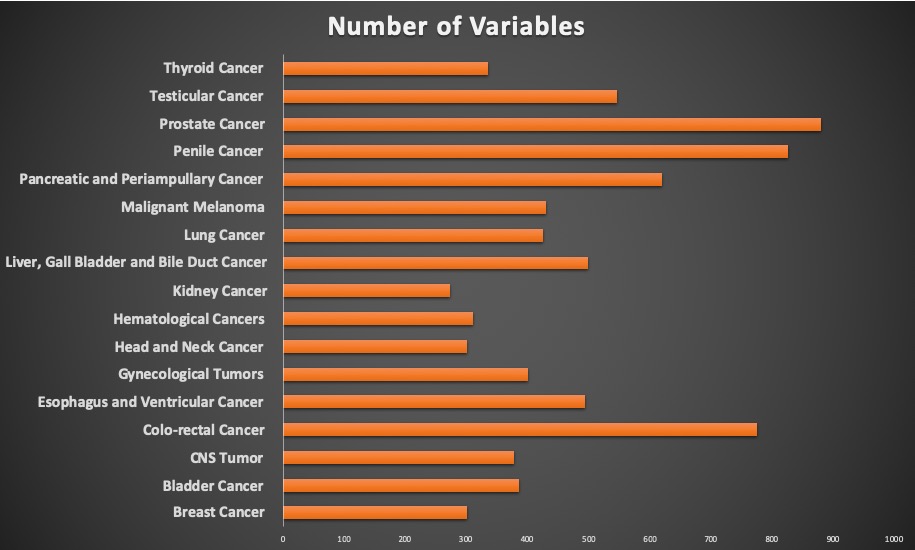
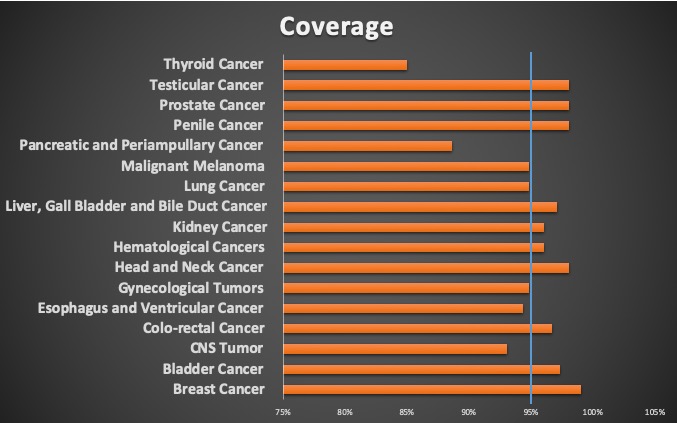
- Coverage
The specific oncology registries have an average coverage around 95% which is more than most of the other registries in other places. The broad coverage is crucial for high quality research by providing the ability of having high volume of patients and vast data points for the studies that are designed. The high coverage is also a demonstration of the broad support of the clinical community towards data collection.
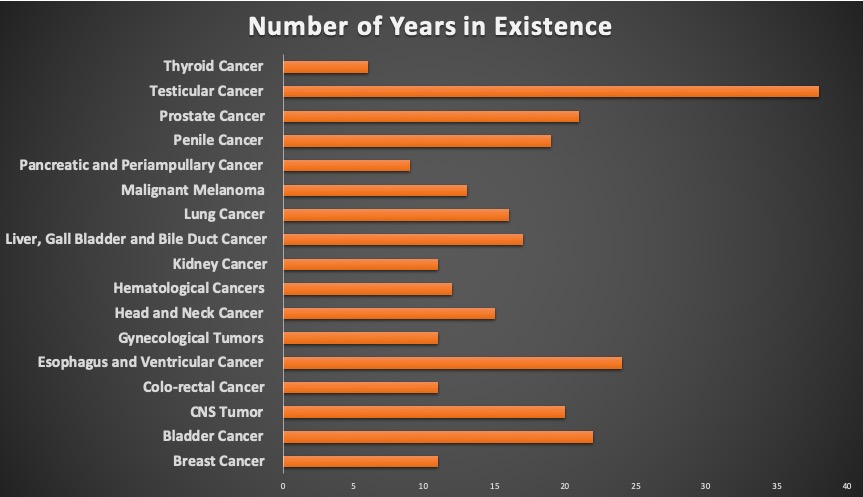
- Duration
Most of the registries have been established for a long time, for an average of 16 years. This has given them the ability to establish a standard practice of data collection and analysis, as well as to give access to a large cohort for longitudinal follow up to assess how different changes in treatment impact the outcomes of interest.
Sweden vs Flatiron
In the consequent blog-posts we are going to evaluate specific examples from published results using data from Flatiron in comparison to the available data in Sweden.
Conclusion
Having considered the basic information that can be extracted from the oncology registries of Sweden, we can already see a huge potential for effective use of the same in reducing the time for regulatory applications and reimbursements. The amount of structured data available and its quality can be applied to effective use for following through to the outcomes and for employment of new treatment practices based on real world evidence.

